is ch4 polar or nonpolar
Is the molecule of ch4 is polar or nonpolar. No CH4 or BeH2 are nonpolar molecules.
 |
| Solved Liilivisuu Iutilii Scilic Question 2 6 2 The Chegg Com |
In CH4 the sharing is equal.
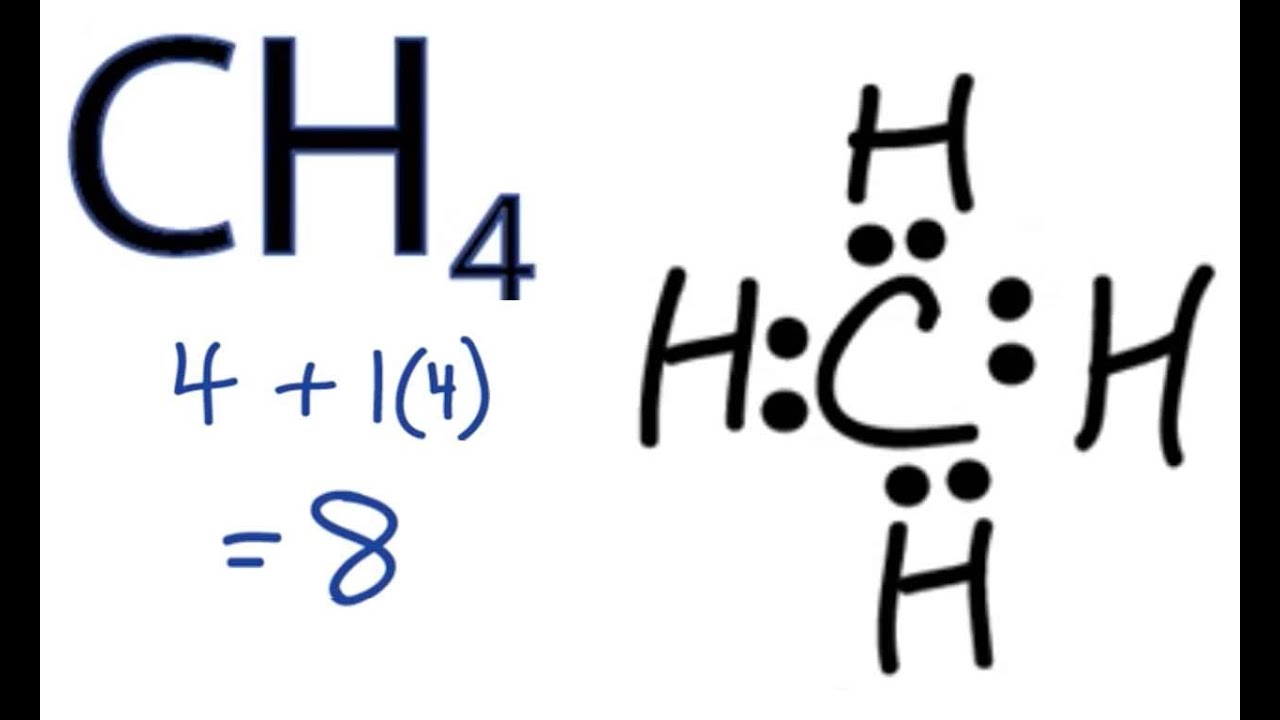
. The shape and geometry of the ion is tetrahedral so that. No2 Polar Or Nonpolar. Here C is a. Also the electronegativity difference of.
CH4 is non polarIt is a tetrahedral compound. Question 20 I found drawings of the lewis structure in many places but all seem to have different answers Cavapoo Puppies. It is colorless odorless and combustible. Yes according to overall discussion we can say that ch4 is nonpolar because this molecules has no any dipole or there is no any poles negative pole or positive pole due to this.
CH4 Methane is a covalent nonpolar covalent compound because when one nonmetal combines with another nonmetal it usually forms a covalent compound. Why is ch4 nonpolar. In case of ch4 molecules there is small difference in electronegativity between carbon and. It has no lone.
All the outer atoms are the same - the same dipoles and that the dipole moments are in the same direction - towards the carbon atom the overall molecule becomes non-polar. The bonds making up the molecule and the shape of the molecule. Let us know about Is NH4 polar or nonpolarThe given chemical species is ammonium ion NH 4 NH 4 and it is a polar compound. Why is CH4 a nonpolar molecule.
How does the Skip to main content close Start your trial now. Build a 3D model if this isnt obvious. Polarity results from an unequal sharing of valence electrons. The electronegativity of carbon and hydrogen.
Therefore CH4 is a nonpolar molecule. Is CH4 polar ionic or nonpolar and list and explain whether it is soluble or insoluble in water. CH4 is a nonpolar molecule due to its symmetrical geometry that causes uniform charge distribution all over the atom leading to a zero net dipole moment and making this molecule. CH4 is a nonpolar molecule because of its symmetric tetrahedral.
CH4 or Methane is a NONPOLAR molecule because all the four bonds C-H bonds are identical and CH4 has symmetrical geometry. So you need to know two facts to judge molecular polarity. The electronegativity of carbon and hydrogen is 255 and 22. Methane CH4 is the simplest hydrocarbon and the main element of natural gas.
While there may be a difference in. So is CH4 polar or nonpolarCH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds. Solution for NH3 vs BH3 NH3 BH3 polar nonpolar polar nonpolar Molecular Geometry Molecular Geometry Question 1b. We know that polar molecules have some dipole moment due to unequal charge distribution but non-polar molecules have an equal charge distribution and.
Atomic mass of each atom Tags. CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds.
 |
| Explain The Non Polarity Of Methane Molecule Ch 4 Homework Study Com |
 |
| Ch4 Lewis Structure Hybridization Molecular Geometry Bond Angle And Shape |
 |
| Methane Molecules Are Nonpolar Molecules Explain Class 11 Chemistry Cbse |
 |
| Which Of The Following Molecules Is Non Polar I Pbci4 Ii Bf3 Iii Sncl2 Iv Cs2 |
 |
| Is Ch4 Polar Or Nonpolar Techiescientist |
Posting Komentar untuk "is ch4 polar or nonpolar"